Who is CMS SciDoc?
CMS SciDoc is your trusted regulatory partner. Our team's deep expertise, international network and proven strategic approach can help you successfully navigate the pathways to global market access. CMS SciDoc is based in Melbourne, Australia and works with companies and organisations across Australia, New Zealand, Asia, USA and Europe.
With extensive experience in regulatory affairs and project management, we manage projects across a diverse range of industries. Our team consists of dedicated industry experts with extensive experience in regulatory affairs, project management and life sciences. We are there to remove the stress and worry out of the entire process of bringing your medical device to market.
Our key values of rapport, integrity, honesty, commitment, attention to detail, efficiency and drive underpin everything we do at CMS SciDoc. We’re highly experienced and we listen, learn, ask questions, and lots of them, after all, we want an in-depth and extensive insight into your project. It’s this understanding that allows us to execute a solution to get your product to market.
Our Expertise
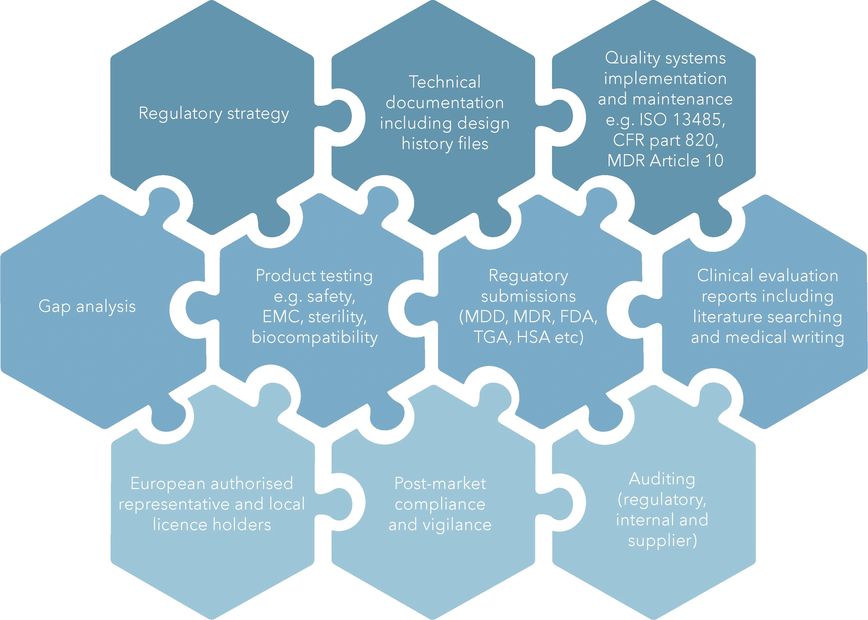
With an extensive history across the life sciences, from start ups, academic institutions, listed biotechs, medtechs and pharmaceutical companies, we understand the complexities of the industry and work closely with you, Notified Bodies and Regulators to get the job done.
